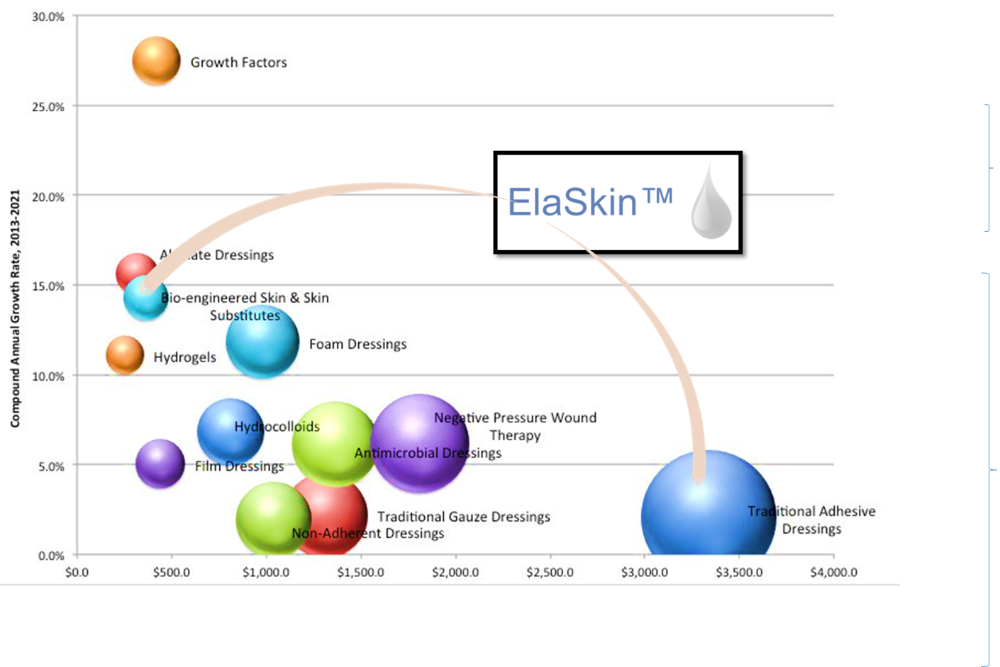
Aleo BME, a leading biomaterials innovator, announced receiving 510(k) market clearance from the U.S. Food and Drug Administration (FDA) for the company's liquid bandage product, ElaSkin™.
The 510(k) clearance allows the company to market and sell the product for use by both healthcare professionals and general consumers.
-lw-scaled.png.png)